From a modern perspective it is very simple. Angiosperms, botanically forming the subclass Magnoliidae (Stevens 2001 onwards), have several feats in common, some of which are synapormophies in a Hennigian sense. Neither Herendeen et al. (2017) nor Wang (2017) provide any concise summary of the characters that can be used to define an angiosperm and to recognise it in the fossil record. Stevens provides such a list from a modern-day perspective (as for all their constituent clades) including some which may be observed in fossil specimens:
- sieve tubes enucleate (in case of perfect preservation)
- stomata brachyparacytic
- fine venation hierarchical-reticulate
- flowers perfect
- anther tetrasporangiate, sporangia in two groups of two, each theca dehiscing longitudinally by a common split, ± embedded in the filament
- endothecium present
- ectexine columellate, endexine lamellate only in the apertural regions
- pollenkit present
- carpels present
- stigma ± decurrent, ovules few [?1]/carpel, anatropous, micropyle endostomal, outer integument 2-3 cells across, inner integument 2-3 cells across
But if we go back in time, and classifications should include fossils, it will be impossible to keep up.
Where all molecular dating studies failed (semantically)
Using a (molecular) branch-based cladistic definition for the angiosperms makes little sense. Based on all currently available data (genes, morphology, fossil record) there can be little doubt that the lineage, which eventually evolved the angiosperms (Magnoliidae), goes back deep in time: the latest phylogenies placed the angiosperms as sisters to all other living seed plants. But there are no flowering plants in the late Palaeozoic or early Mesozoic. Nothing showing even a single of the uniquely shared traits (synapomorphies) characterising the Magnoliidae. Maybe with one exception: Hochuli & Feist-Burkhardt's (2004, 2013) infamous “angiosperm-like” pollen [BTW: It's “angiosperm-like” because it is an angiosperm-type pollen, but from the Triassic.]175 million years are a long time to evolve and become something else (e.g. a flowering plant). The first member of the angiosperm clade (technically: the class Magnoliopsida) was likely not only the ancestor of angiosperms, but also of an unknown number of Permian and Mesozoic seed plants. It and most of its early descendants did probably not possess the diagnostic set of angiosperm characters. Predating or coeval with the first “angiosperm-like” Triassic pollen are seed plants of unknown affinity, colloquially addressed as ‘seed ferns’. Some of which are morphologically quite advanced. The dominant seed fern group popping up in the Triassic in the mid-latitudes of the Northern Hemisphere (30–60 °N; thanks to C. Pott for spotting my earlier error) are the bennettites (Bennettitales) with flower-analogue structures (the Jurassic is not the “Age of Cycads” but the “Age of Bennettites”; for contemporary research on bennettites covering various aspects see the many papers by Christian Pott [GoogleScholar/ResearchGate]). In the Permian of the Southern Hemisphere, we have the glossopterids (Glossopteridales; see e.g. publications by Stephen McLoughlin [GS/RG]) as the dominant floral element, equally unplaced. They go essentially extinct during the PT-event, at least 75 myrs after the inferred split between gymnosperms and angiosperms. In the Triassic of the Southern Hemisphere, we then find the only current candidate for an actual extinct sister lineage of the angiosperms, the Petriellales.
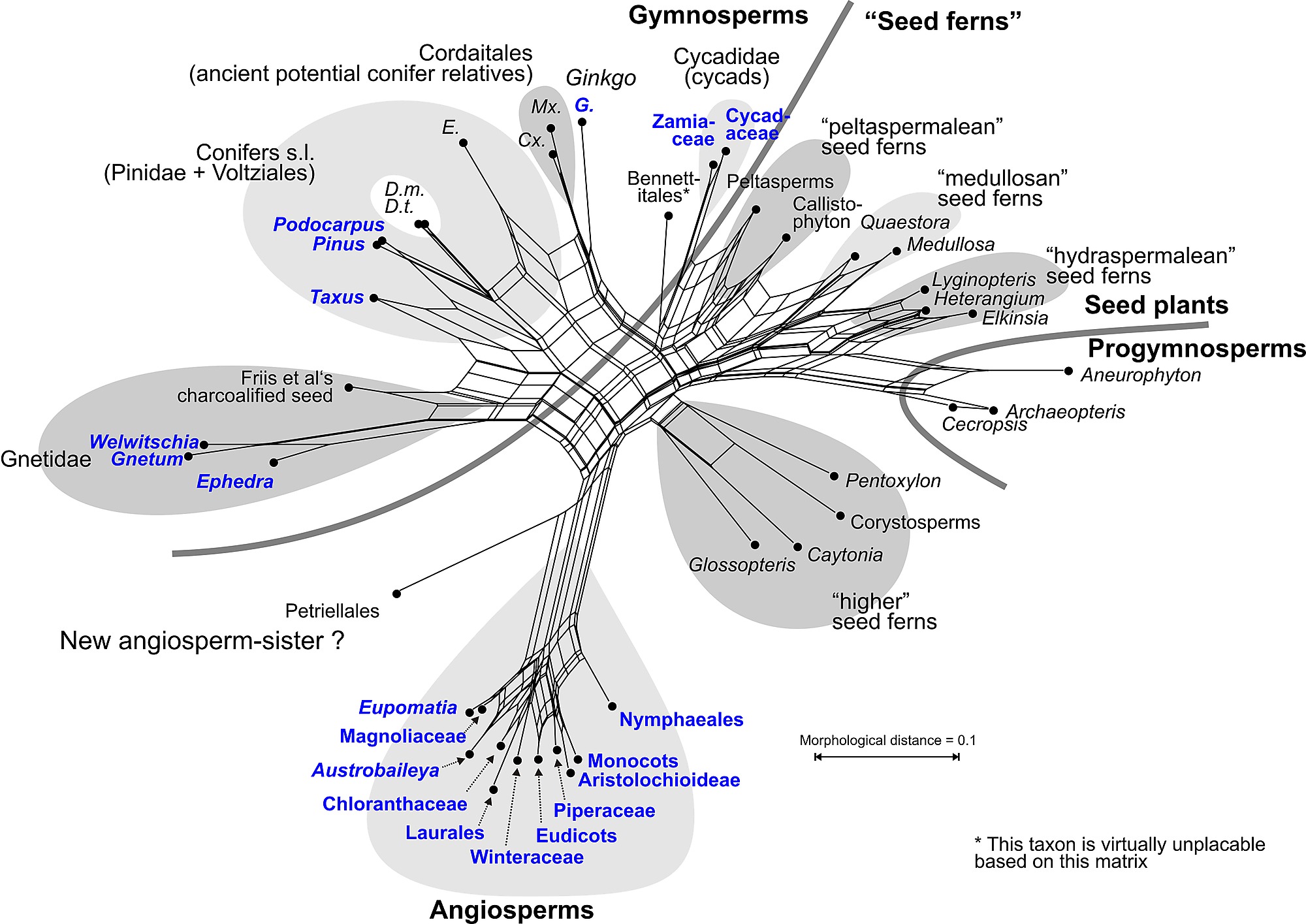 |
| Morphology-based phylogenetic relationships between extant (blue font) and extinct seed plants based on the most recent data matrix by Rothwell & Stockey, 2016). @figshare; @Genealogical World of Networks |
With respect to the length of the angiosperm root in dated (above) or non-dated molecular trees (see also this post), it is a safe bet that the angiosperms were a relative late addition to this obscure seed plant lineage living in the shadow of their distant sisters, the gymnosperms. And the only Magnoliopsida successful enough to undergo a massive radiation and diversification; obviously, after they evolved a favourable set of highly competitive traits including a perfect flower. Possibly Late Jurassic, we should give them some time to radiate and diversify (none of the angiosperms in the Early Cretaceous is thoroughly primitive; they all combined primitive and derived angiosperm traits).
Short digression on Permian or Triassic angiosperm crown-group radiation (in case molecular daters read this post)
Not interested, skipIn the above context, Late Palaeozoic or early Mesozoic estimates for crown-group radiation, i.e. radiation of modern-day Magnoliidae, make no sense from an evolutionary point of view. They are methodological artefacts of the Bayesian relaxed clocks used to generate them. The devil lies in the position of the available ‘safe’ age constraints coupled with a very pronounced angiosperm root, followed by a very shallow radiation, nodes known as the “Dirty Dozen”. Herendeen et al. (2017) argued that we should only accept those fossils as “crown group angiosperms” that can be reliably linked to one of the modern angiosperm lineages (a poly-phyletic definition). Being higher up in the tree and in lineages with varying substitution rates, all those fossils will inevitably lead to (much too) old estimates for the primary divergences. Well demonstrated by a recent study by Salomo et al. (2017) doing a series of tests.
- link the fossil to a deeper node (“conservative treatment”)
- reduce the taxon sample to a set that the node corresponding to the fossil is replaced by a much deeper one (for instance, Salomo et al. used various crown-group fossils of several lineages within the magnoliids, a lineage well-presented in their taxon set; by reducing the number of magnoliid representatives, we have to move them to deeper nodes, hence, get younger estimates)
- They weren't (yet) angiosperms, i.e. they did not have any of the unique, shared, derived traits of modern-day angiosperms.
- If they were, they did not grow close to water bodies, but in deserts or similar extreme habitats that allow no fossilisation.
The problem with explanation 2 and old estimates is that such extreme environments are usually not the cradle of a large, dominating group of organisms, but home of few late specialists of such a large group (angiosperm succulents are deeply nested in the angiosperm tree), or the last survivors of a once-dominating or much more common group (like the oddest desert plant: the Gnetidae Welwitschia, a living fossil).
Thus, the only explanation for Darwin's ‘Abominable Cretaceous Mystery’ is that the angiosperm precursors were nothing close to modern-day angiosperms. The accumulation of the angiosperm-unique traits were obviously the reason angiosperms quickly diversified and conquered the world in the Early Cretaceous (see maps in Chaboureau et al. 2014). Reflected by pronounced root and shallow but broad basal radiation.
From cladistic to phylogenetic classification
In Part 1, I already pointed out that node-based (tree-naïve) classifications will often be non-Hennigian when fossils should be included.But in the case of angiosperms, we don't need to infer a fossil-inclusive phylogeny. We have a lot of morphological traits associated with the crown node/clade (and subclades: modern angiosperm lineages). We can use them to morphologically characterise the node corresponding to the MRCA of all extant angiosperms, by comparison or reconstruction as done by Sauquet, von Balthazar & Schönenberger (2017) for the flower. The problem is that we have no idea about the time, place, and mode – when, where, and how – the angiosperms obtained their synapomorphies. Welcome to Farris' Uncertainty Zone (FUZ).
 |
| Farris' Uncertainty Zone. It is likely that late precursors and sister lineages share the characteristics of a modern-day clade. |
If the FUZ is small a cladistic and phylogenetic classification will be synonymous, and we can use individual traits to identify the earliest members of our clade/holophylum. But if the FUZ is large, we have an unknown number of things that look like an angiosperm, but are not an angiosperm according to our node-based definition, because they are not descendants of the MRCA of the extant angiosperms, but ancestors or extinct sisters.
The only solution is to use a phylogenetic classification. Any organism that has the set of derived traits shared uniquely by modern angiosperms is an angiosperm – under the assumption backed by molecular phylogenies that such unique trait sets evolved only once, i.e. reflects the inclusive common origin (holophyly). And that all (early) descendants of the common ancestor shared those traits (originally, later some may get lost or modified; inevitably so, the beauty is called evolution).
Can we keep up with Hennig's ideal?
Noting the unambiguity of the angiosperm clade and the relative large set of shared derived traits (including some synapomorphies), it is tempting to use a phylogenetic classification following Hennig's rules naming only holophyla, groups of inclusive common origin.- Define the angiosperms (Magnoliidae) as all descendants of the last common ancestor (but not the MRCA of modern members) that shows the suite of traits uniquely shared by all extant angiosperms (Option 1).
- Define a higher rank taxon (Magnoliopsida) that includes the angiosperms and their extinct potential relatives (e.g. the Petriellales) until the first common ancestor, the point at which the Magnoliopsida diverged from the gymnosperms (Gymnospermopsida).
- Define the modern (crown) angiosperms (Magnoliinae) as the Magnoliidae above;
- Define the angiosperms (Magnoliidae) as all descendants of the first organisms hosting at least one trait diagnostic for modern angiosperms.
 |
| Hennig's Ambiguity Zone. Fossils c, x, and y may be A-idae or sister lineages/precursors. Given we have sensible molecular age estimates, we can infer that c is an A-idae as is shows a diagnostic feature found also in the A-idae A and b and is younger than the A-idae MRCA (keeping in mind that reliable node-dating estimates will typically be underestimating/are minimum ages) |
On the other hand, why should one diagnostic, uniquely derived character not be enough (Option 2)? For the example above, x and y can be classified as A-idae as they show traits exclusively found in the A-idae, and only z would be ambiguous. Which brings us back again to the Triassic “angiosperm-like” pollen and the Petriellales, a potential but distinct sister group. But there is little in-between serving as a Option-2-Magnoliidae (mainly pollen, a lot unpublished thanks to the mechanics of the Impermeable Fog – confidential peer review), but then a lot in the mid-Cretaceous serving as Option-1-or-2-Magnoliidae [Most of the discussion revolves around things like is there a double integument (= Option-1-Magnoliidae) or not (= Option-2-Magnoliidae).] But: There are so far no-clear-2-synapomorphies-evolved-x-to-go taxa. Currently, the angiosperm HAZ covers quite a time. And we can live with it.
How to classify an angiosperm
It would be plain-stupid to not make use of the rare coincidence that morphologies fully match molecular data, hence use a Hennigian, largely synapomorphy-based, definition of angiosperms (Option 1) – holophyletic Magnoliidae. Define characters or a suite of characters unique to extant (crown-group) angiosperms that can be traced in the fossil record.With respect to unknown extension of the FUZ and HAZ, and yet to be found ancestor and extinct (more or less distant) sister lineages of the angiosperms, a Hennigian phylogenetic classification will however be doomed (instable, overly complex or indiscriminate). The workaround is to define up to three paraphyletic taxonomic groups:
- the literally basal angiosperms (subgroup of the holophyletic Magnoliidae) – early crown-group angiosperms resolved as sisters or potential precursors of more than one modern clade;
- the proto-angiosperms (same rank as Magnoliidae) – the actual stem angiosperms, i.e. the (likely) direct precursors of angiosperms and their extinct offspring;
- the angiospermoids (same rank as Magnoliidae) for all other primordial, early diverging and difficult to place members of the deep-rooting angiosperm-lineage, the holophyletic Magnoliopsida.
 |
| Concept for a Haeckelian phylogenetic classification of angiosperms and their relatives. |
Distinction between angiosperms, proto-angiosperms, angiospermoids, and non-Magnoliopsida could be straigthforward:
Regarding angiosperm dating, it would make more sense to talk of an angiospermoid or Magnoliopsida stem age, because the actual angiosperm stem age – divergence between (proto-)angiosperms and their closest (last) proto-angiosperm (angiospermoid) sister clade cannot be estimated using only modern-day molecular data. We lack survivors of the sister groups. The divergence between the (proto-)angiosperms and their closest (last) sister clade was probably not Permian, but somewhere in-between the ‘angiosperm’ root node and the (actual) angiosperm crown node in molecular trees. A strange co-incidence is that the mid-point of the angiosperm root branch in dating studies with medium-young angiosperm crown ages falls in the time of the first Petriellales (see above). Maybe, this is the actual time when the proto-angiosperms isolated from other Magnoliopsida, evolved their first unique trait (“angiosperm-like” pollen). And survived.
Not restricting the classification to holophyla may further pay off in future, because we have, so far, no conclusive evidence that the modern-day angiosperm synapomorphies have been synapomorphies in the past. One explanation for Darwin's “Abominable Mystery” would be hybrid vigour and incomplete lineage (trait) sorting in a fast radiating and quickly diverging group. Several closely related (everything that today forms a holophyletic order or more started with a single species/genus) proto-angiosperm lineages may have evolved the capacity to produce one of the traits that what we now recognise as a synapormorphy. Since each of those innovations were generally beneficial, a combination of them would have been highly beneficial.
Furthermore, sublineages expressing the new traits would have been much more competitive than those not expressing it. One can imagine that during the initial radiation there were many species/lineages that kept the one or other primitive trait, but soon went extinct. Multiple origins within a dynamic proto-angiosperm group of still closely related species/genera would explain why early (truly basal) angiosperms have combinations of derived traits found in more than one modern lineage (making total-evidence dating impossible).
Related posts
On angiosperms
-
What is an angiosperm? Part 1: The difference between cladistic and phylogenetic classification.
Me @ Res.I.P., January 2018.
-
Age of angiosperms, may palaeobotany rest in peace (and pieces). Me @ Res.I.P., December 2017.
-
What I was not allowed to show #1: A neighbour-net of seed plants. Me @ Res.I.P., October 2017.
-
Should we try to infer trees on tree-unlikely matrices? Me @ GWoN, July 2017.
-
Summarizing non-trivial Bayesian tree samples for dating? Just use support consensus networks. Me @ Genealogical World of Networks (GWoN), January 2018.
- The most common errors regarding node dating. Me @ Res.I.P., December 2017.
-
Networks, not trees, identify "weak spots" in phylogenetic trees. Me @ GWoN, October 2017.
-
More non-treelike data forced into trees: a glimpse into the dinosaurs. Me @ GWoN, August 2017.
- Two papers you may want to read before inferring trees based on morphological data. Me @ Res.I.P., September 2017.
-
Let's distinguish between Hennig and Cladistics. David Morrison @ GWoN, October 2017.
-
Clade, cladograms, cladistics, and why networks are inevitable. Me @ GWoN, October 2017.
- Phylogenetic networks 1900-1990. David Morrison @ GWoN, September 2013. To think about alternatives and reticulation is not new.
- Is there a philosophy of phylogenetic networks? David Morrison @ GWoN, February 2013
Postscriptum for specialists: Some examples for how it could work
(just some ideas that popped into my head, obviously, real palaeobotanists have to make the final calls)Pending a proper (in-depth SEM) investigation of the dispersed Triassic and Jurassic pollen record, I would include all dispersed angiosperm-like pollen in the proto-angiosperms. Until it can be shown that sculptured monosulcate pollen can be found in extinct groups that are not angiosperms or proto-angiosperm, hence, that there is proof that it is a symplesiomorphy (a shared primitive trait) or parallel mutation (evolved in more than one lineage) of the Magnoliopsida in general. In any case, angiosperm-like pollen will be the main puzzle piece to identify members of the Magnoliopsida. Gymnosperms obviously never evolved such pollen, not even the most derived (genetically and morphologically) Gnetidae.
Based on the morphological data set compiled by Rothwell & Stockey (2016) and subsequent phylogenetic analysis (Coiro, Chomicki & Doyle 2017; Grimm 2017a [post]), the Petriellales (Bomfleur et al. 2014) are so far the only definite candidate for an angiospermoid (it will be most interesting to see their pollen at one point). Should they stabilise as last angiosperm sister clade (sister to proto-angiosperms), we may want to call them Petriellidae.
Caytonia may come back into the club in the future, because the alternative is that Caytonia is a gymnosperm. With the current molecular framework and its placement in comprehensive morpho-phylogenetic analysis, it's either the one or the other. There is little place for a third independent, late and derived lineage of seed plants. So dear palaeobotanical experts, make your pick: gymnosperm or Magnoliopsida/angiospermoid.
Further candidates for angiospermoids are the Erdtmanithecales (or part of them, see Rothwell, Crepet & Stockey's 2009 critique of Friis et al. 2007; the in-text arguments, please ignore all “cladistic analyses and tests”) in case their pollen is found to be “angiosperm-like” as insinuated by Herendeen et al. The don’t seem to be angiosperm-precursor, thus finding the association of an “angiosperm-like” pollen with these plants would mean that such pollen is not unique to the Magnoliidae but a Magnoliopsida symplesiomorphy (shared ancestral) or parallelism (shared derived trait).
No-one can – at this point – say whether the Bennetittales or Glossopteridales are gymnosperms or Magnoliopsida. Bennetittales pollen is similar to cycad pollen, otherwise they have very little in common. Cycads are either part of the gymnosperm lineage or a very distant sister lineage of angiosperms (a pre-angiospermoid divergence). However, there is no reason to assume that such uncharacteristic pollen is not a primitive trait of (higher) seed plants. Both groups appeared after the assumed gymnosperm-Magnoliopsida split on Earth's stage, so they may represent early radiations of either lineage or a third major seed plant lineage.
The Gnetidae are not Magnoliopsida, but likely gymnosperms. Their placement as sister to the angiosperms in morphology-based reconstructions (e.g. Hilton & Bateman 2006; Friis et al. 2007, but see Grimm 2017b and the related post; Rothwell et al. 2009; Rothwell & Stockey 2016, but see Coiro et al. 2017) is a long-branching artefact, just like their placement as sister to the Pinaceae in early, plastid-data dominated molecular trees (the Pinales/Pinitidae are likely holophyletic). All molecular data put them (consistently) closer to Ginkgo and the Pinitidae (the conifers) root(s) than to the angiosperms; and even if we force them back to the Magnoliopsida, they would not end up within the lineage but be placed as a very distant sister, morphologically and genetically (a bit like the extremely short-branched cycads in some earlier trees; cf. Mathews 2009)
Thanks to Benjamin Bomfleur, Stephen McLoughlin, Christian Pott, Jim Smith and Stefan Wanke for quickly answering some questions/requests that came to my mind while doing this 2-part post.
References
Bomfleur B, Decombeix A-L, Schwendemann AB, Escapa IH, Taylor EL, Taylor TN, McLoughlin S. 2014. Habit and ecology of the Petriellales, an unusual group of seed plants from the Triassic of Gondwana. International Journal of Plant Sciences 175:1062–1075.Chaboureau A-C, Sepulchre P, Donnadieu Y, Franc A. 2014. Tectonic-driven climate change and the diversification of angiosperms. Proceedings of the National Academy of Sciences 111:14066–14070.
Cohen KM, Finney SC, Gibbard PL, Fan J-X. 2013 (updated). The ICS International Chronostratigraphic Chart. Episodes 36:199–204. http://www.stratigraphy.org/index.php/ics-chart-timescale
Coiro M, Chomicki G, Doyle JA. 2017. Experimental signal dissection and method sensitivity analyses reaffirm the potential of fossils and morphology in the resolution of seed plant phylogeny. bioRxiv DOI:10.1101/134262 http://biorxiv.org/content/early/2017/06/07/134262
Earle CJ. 2010. The Gymnosperm Database. http://www.conifers.org/.
Grimm G. 2017a. Morphology-based neighbour-net of seed plants: quick exploratory data analysis of the matrix of Rothwell & Stockey (2016). figshare. https://doi.org/10.6084/m9.figshare.5143732.v1
Grimm GW. 2017b. Morphology-based neighbour-net of seed plants. figshare. https://doi.org/10.6084/m9.figshare.5111062.v1
Herendeen PS, Friis EM, Pedersen KR, Crane PR. 2017. Palaeobotanical redux: revisiting the age of the angiosperms. Nature Plants 3, article no. 17015. dx.doi.org/10.1038/nplants.2017.15
Hilton J, Bateman RM. 2006. Pteridosperms are the backbone of seed-plant phylogeny. Journal of the Torrey Botanical Society 133:119-168.
Hochuli PA, Feist-Burkhardt S. 2004. A boreal early cradle of angiosperms? Angiosperm-like pollen from the Middle Triassic of the Barents Sea (Norway). Journal of Micropalaeontology 23:97–104.
Hochuli PA, Feist-Burkhardt S. 2013. Angiosperm-like pollen and Afropollis from the Middle Triassic (Anisian) of the Germanic Basin (Northern Switzerland). Frontiers in Plant Science DOI:10.3389/fpls.2013.00344.
Magallón S, Gómez-Acevedo S, Sánchez-Reyes LL, Hernández-Hernández T. 2015. A metacalibrated time-tree documents the early rise of floweringplant phylogenetic diversity. New Phytologist 207:437–453.
Mathews S. 2009. Phylogenetic relationships among seed plants: Persistent questions and the limits of molecular data. American Journal of Botany 96:228–236.
Rothwell GW, Crepet WL, Stockey RA. 2009. Is the anthophyte hypothesis alive and well? New evidence from the reproductive structures of Bennettitales. American Journal of Botany 96:296–322.
Rothwell GW, Stockey RA. 2016. Phylogenetic diversification of Early Cretaceous seed plants: The compound seed cone of Doylea tetrahedrasperma. American Journal of Botany 103:923–937.
Salomo K, Smith JF, Feild TS, Samain M-S, Bond L, Davidson C, Zimmers J, Neinhuis C, Wanke S. 2017. The emergence of earliest angiosperms may be earlier than fossil evidence indicates. Systematic Botany 42:1–13.
Sauquet H, von Balthazar M, Schönenberger J. 2017. The ancestral flower of angiosperms and its early diversification. Nature Communications 8, article no. 16047. https://www.nature.com/articles/ncomms16047
Stevens PF. 2001 onwards. Angiosperm Phylogeny Website. Version 8, June 2007 [and more or less continuously updated since]. Available at http://www.mobot.org/MOBOT/research/APweb/ (accessed 30/03/2017.
Wang X. 2017. A biased, misleading review on early angiosperms. Natural Science 9:399–405. https://doi.org/10.4236/ns.2017.912037





No comments:
Post a Comment
Enter your comment ...